carboxylate ir stretch
We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions. Furthermore we have shown.
Lets compare the strength of that bond to.
. Alkane C-H bonds are fairly ubiquitous and therefore usually less useful in determining structure. Give a band in the IR spectrum if it is accompanied by a change of. We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions.
We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions. Several studies have considered the M 2-induced spectral response of carboxylates in various chemical environments 19 2330In anhydrous crystals for example. The weight of the IR band area using the COO.
Gas-phase spectra of a. A bond vibration like stretching will only be IR-active ie. So in magenta this is the nitrogen-hydrogen bond stretch.
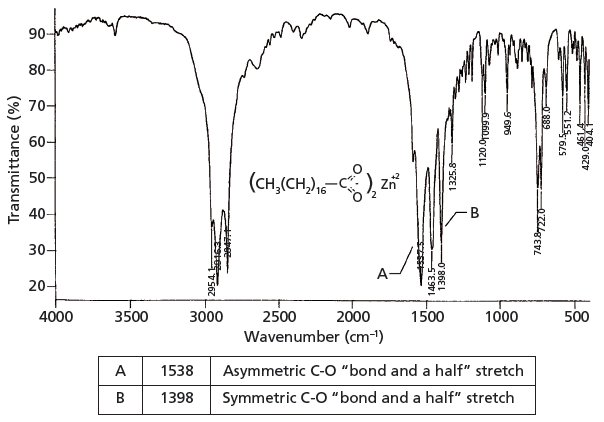
Infrared monolayers spectra of the metal-carboxylate complexes and the wavenumbers of the symmetric and antisymmetric vibrational modes are reported. A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is. The surface composition and morphology of the TiO 2 coated HDG-steel substrate have been in detail previously.
Gas-phase spectra of a series of. A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate. It is reported that the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions is reported.
Abstract A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the. Table of IR Absorptions. We have tested this principle with ab initio modeling for aqueous.
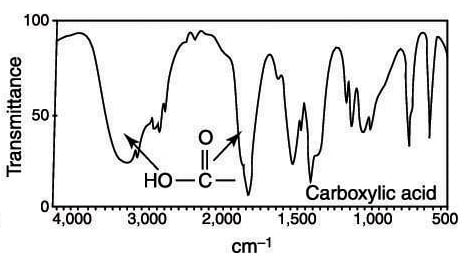
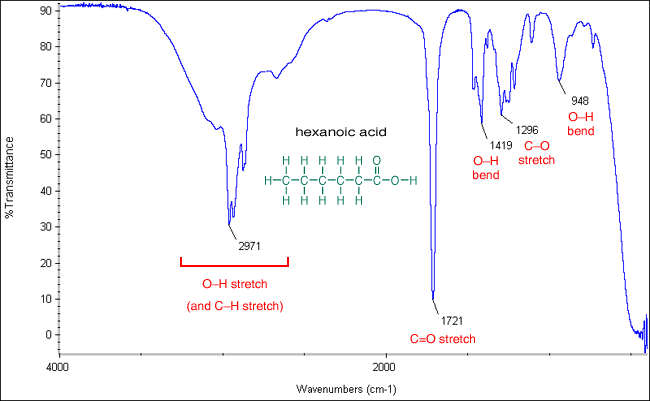
The carbonyl stretching bands were the most distinctive IR features to distinguish the carboxylic acid group from the conjugate carboxylate moiety 41 42. This is the bond stretch for nitrogen-hydrogen so thats this bond stretching right there. Absorption peaks above 3000 cm -1 are frequently diagnostic of.
A linear standard curve using anhydrous sodium salicylate has been obtained from the plot of carboxylate COO concentration vs. Carboxylate anions exhibit characteristic vibrational spectra in the infrared IR which typically includes an intense peak due to symmetric stretching of the COO functional. When the bonds of the -CO 2 group stretch asymmetrically and symmetrically there is a large value of dµdx.
Gas-phase spectra of a. 24 After deposition of R 12 C the ATR-IR data show CH. The carbonyl group of an ester therefore has a C-O double-bond character than does the carbonyl group of a ketone so the former is stronger and harder to stretch.
In comparison with its corresponding free ion a large splitting of the co 2 stretching frequencies δ ν is often an indication of monodentate coordination in a metal carboxylate. Carboxylate ions can be formed by deprotonation of carboxylic acids. This attribute is why the pair of carboxylate stretching peaks are.
Such acids typically have p Ka of less than 5 meaning that they can be deprotonated by many bases such as sodium. We report the first IR.

Pdf Infrared Spectroscopy Of Metal Carboxylates Ii Analysis Of Fe Iii Ni And Zn Carboxylate Solutions

Ir Spectra Of Indole 3 Acetic Acid In Kbr Indole Nh Band At 3389 Cm 1 Download Scientific Diagram
The C O Bond Part Iii Carboxylic Acids
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

Infrared Spectrum Of Ethanoic Acid Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Ethanoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes
How To Identify Carbonyls Alkenes Alkynes And Aromatics In The Ir Spectrum Dummies

Ft Ir Spectrum Of Poly Ethylene Oxide Urethane Dimethacrylate With Download Scientific Diagram

Infrared Spectrum Of Butanoic Acid C4h8o2 Ch3ch2ch2cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Butyric Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes

The Carbonyl Group Part V Carboxylates Coming Clean
20 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

Synthesis And Characterization Of Cu Ii Zn Ii And Sm Iii Metal Complexes With Itaconic Acid
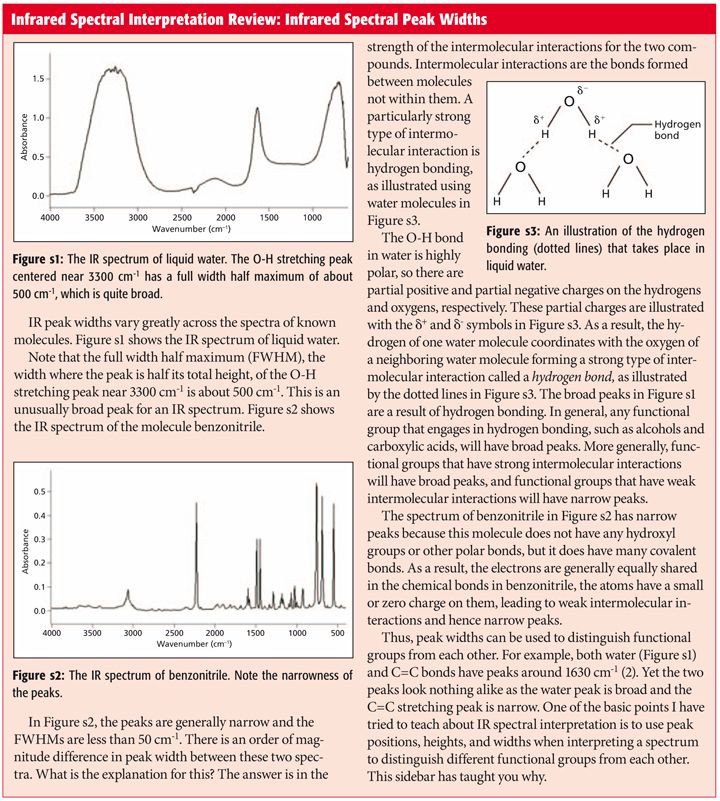
The C O Bond Part Iii Carboxylic Acids
The C O Bond Part Iii Carboxylic Acids

Infrared Spectrum Of Benzoic Acid C7h6o2 C6h5cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Benzoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes

Interpretation Of Carboxylic Acid Ir Spectra Youtube
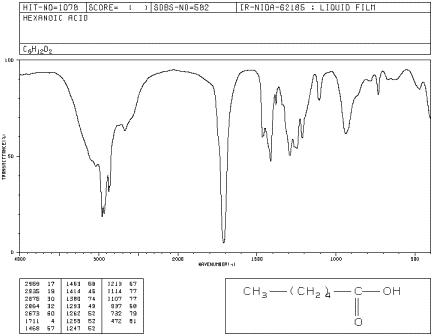


Comments
Post a Comment